The 2025 AHA Advanced Life Support Guidelines: Raising the Standard of Resuscitation Care

The 2025 AHA Advanced Life Support Guidelines: Raising the Standard of Resuscitation Care
DISCLAIMER
This blog contains discussions and general information about medical emergencies and other health topics. The information in this blog and in other related resources is not meant to be medical advice, and it should not be taken as such. The information contained in this blog should not be used in place of a medical doctor’s advice or treatment. If you or someone else has a medical concern, you should talk to your doctor or seek out other professional medical care. The opinions and views on this blog and website have no connection to those of any school, hospital, medical facility, or other organization.
Highlights of the 2025 American Heart Association Guidelines
A New Era of Evidence-Based Resuscitation
On October 22nd 2025, the American Heart Association (AHA) released its updated Guidelines for Cardiopulmonary Resuscitation (CPR) and Emergency Cardiovascular Care (ECC). Among these, the Part 9: Adult Advanced Life Support (ALS) guidelines stand out for reshaping how healthcare providers respond to cardiac arrest, shockable rhythms, and life-threatening arrhythmias.
For doctors, nurses, and paramedics, these updates form the cornerstone of Advanced Cardiovascular Life Support (ACLS) training—empowering teams to improve outcomes through evidence-based, system-driven care.
Key recommendations from the 2025 ACLS Guidelines – Algorithms
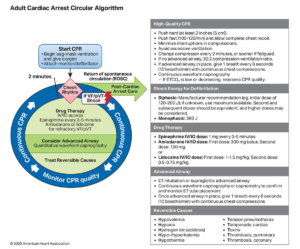
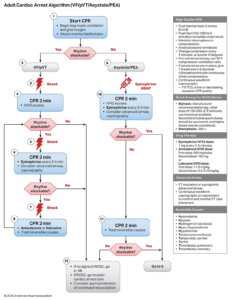
Defibrillation in Adult Cardiac Arrest — 2025 AHA ACLS Guidelines
The 2025 AHA Advanced Life Support Guidelines reaffirm defibrillation as a critical intervention for ventricular fibrillation (VF) and pulseless ventricular tachycardia (pVT) during cardiac arrest.

Key recommendations:
Defibrillation
- Defibrillation is essential for all shockable rhythms (VF/pVT).
- Biphasic defibrillators are preferred over monophasic models for improved safety and efficacy.
- A single-shock strategy is recommended to minimize CPR interruptions and enhance survival.
- Energy settings should follow manufacturer guidelines — using either fixed or escalating energy levels depending on device type.
- If the first shock is unsuccessful, increasing energy for subsequent shocks is reasonable.
- When unsure of the correct setting, use the manufacturer’s default or maximum energy for the first shock.
- In short, early defibrillation with appropriate energy selection and uninterrupted CPR remains vital to improving outcomes in cardiac arrest management.
Ancillary Waveform Technology
During cardiopulmonary resuscitation (CPR), continuous chest compressions create electrical “noise” on the ECG, known as artifact, which can obscure the patient’s actual cardiac rhythm. This makes rhythm interpretation challenging and may lead to delayed or inappropriate interventions. To address this, researchers have explored new technologies such as artifact-filtering algorithms and ventricular fibrillation (VF) waveform analysis to interpret rhythms without interrupting compressions. However, current evidence does not yet support their routine clinical use.
| # | Key Recommendation | Summary |
| 1 | The effectiveness of artifact-filtering algorithms for rhythm analysis during chest compressions has not been established. | These technologies cannot yet reliably identify cardiac rhythms during ongoing CPR. |
| 2 | The effectiveness of VF waveform analysis to guide cardiac arrest management has not been established. | Using VF waveform characteristics to guide shock or medication decisions is not supported by current evidence. |
Clinical Context
Both technologies aim to reduce interruptions in CPR and improve real-time rhythm interpretation. Despite their potential, studies to date have been inconclusive, and their accuracy, safety, and clinical benefit remain unproven. Until further validation occurs, their use should be limited to research or monitored trials.
Clinical Takeaway
- Do not rely on artifact-filtering or waveform analysis tools for decision-making during CPR.
- Continue using standard rhythm checks at appropriate intervals while minimizing pauses.
- Prioritize high-quality, continuous chest compressions and timely defibrillation — interventions proven to improve survival.
Vector Change and Double Sequential Defibrillation
When a patient remains in ventricular fibrillation (VF) or pulseless ventricular tachycardia (pVT) after several shocks, clinicians sometimes attempt alternative strategies such as changing pad position (vector change) or using two defibrillators in sequence (double sequential defibrillation – DSD). The 2025 AHA Guidelines reviewed the available evidence and found no clear clinical benefit for either method at this time.
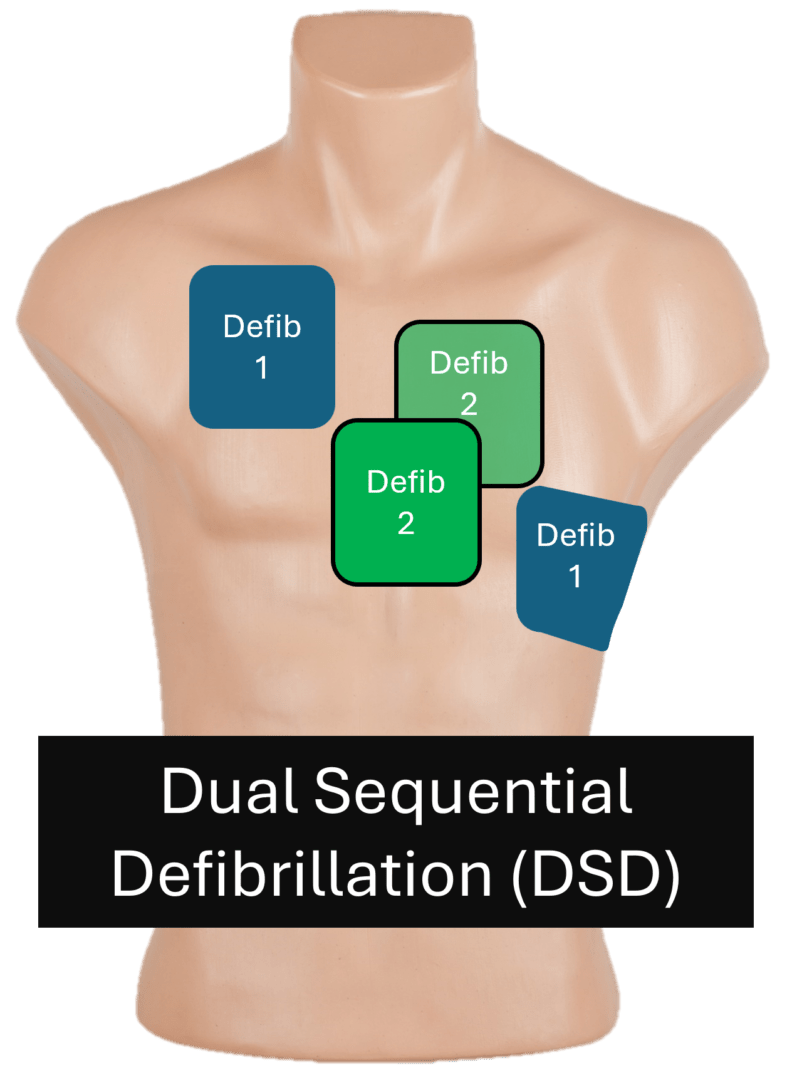
| # | Key Recommendation | Summary |
| 1 | The usefulness of changing pad position (vector change) after three or more unsuccessful shocks has not been established. | Evidence does not show improved survival or rhythm conversion with pad repositioning. |
| 2 | The usefulness of double sequential defibrillation (DSD) for persistent VF/pVT after three or more shocks has not been established. | No proven advantage over standard defibrillation; timing and technique remain inconsistent. |
Clinical Context
Effective defibrillation depends not only on energy settings but also on factors like pad placement, contact quality, and minimal interruption in CPR.
The terms “shock-resistant” or “shock-refractory” VF/pVT are often used, but the guidelines now prefer “persisting VF/pVT” because many cases thought to be refractory are actually recurrent VF following temporary rhythm restoration.
Before attempting vector change or DSD, rescuers should optimize existing technique — ensuring proper pad placement, strong skin contact, and continued high-quality CPR.
Clinical Takeaway
- Vector change and double sequential defibrillation remain experimental and should not replace standard defibrillation practices.
- Focus on the basics: correct pad placement, adequate energy selection, and high-quality, uninterrupted CPR.
- Recognize that most “refractory” VF is actually recurrent, and may respond better to antiarrhythmic drugs or post-shock stabilization, not additional shock techniques.
Nondefibrillation Electrical Therapies
Besides defibrillation, other electrical interventions — such as transcutaneous pacing or mechanical alternatives like cough CPR and precordial thump — have been evaluated for use during cardiac arrest. The 2025 AHA Guidelines confirm that these methods do not improve survival and should not be used as routine interventions during established cardiac arrest.

| # | Key Recommendation | Summary |
| 1 | Routine use of electrical pacing is not recommended during resuscitation of adult cardiac arrest. | Studies show no improvement in return of spontaneous circulation (ROSC) or survival outcomes with pacing during cardiac arrest. |
Clinical Context
Transcutaneous pacing theoretically stimulates cardiac contractions, but clinical trials have consistently shown no benefit when used in established cardiac arrest.
Other interventions such as cough CPR, fist or percussion pacing, and the precordial thump may be considered only in specific peri-arrest scenarios — for instance, an immediately witnessed arrest where advanced interventions are not yet available.
Outside of those rare, early moments, these techniques delay proven interventions such as CPR and defibrillation.
Clinical Takeaway
- Do not perform electrical pacing during cardiac arrest — it offers no proven benefit.
- Focus on interventions that improve outcomes: high-quality CPR, timely defibrillation, and effective airway management.
- Reserve manoeuvres like precordial thump or cough CPR only for immediate, witnessed arrests while awaiting defibrillation.
Vascular Access in Cardiac Arrest Management
During cardiac arrest, timely vascular access is essential for drug delivery and fluid administration. The 2025 AHA Guidelines reaffirm that intravenous (IV) access remains the preferred route, with intraosseous (IO) access as an effective alternative when IV placement is delayed or not possible. Central venous access may be considered in select hospital settings by trained professionals.
| # | Key Recommendation | Summary |
| 1 | Establish IV access as the first choice for drug administration during cardiac arrest. | IV remains the preferred and most effective route for medication delivery. |
| 2 | Use IO access if IV attempts are unsuccessful or not feasible. | IO access provides a rapid and reliable alternative, particularly in prehospital settings. |
| 3 | Consider central venous access only if both IV and IO routes are unsuccessful and a trained provider is available. | Central lines should not delay resuscitation and are mainly appropriate in hospital environments. |
Clinical Context
IV access has long been the standard for administering emergency medications. However, securing a vein during cardiac arrest can be difficult due to poor perfusion or collapsed vessels. IO access—through the tibia or humerus—offers a fast, effective alternative with similar drug absorption and circulation times.
Central venous lines may deliver drugs more directly to the heart but require advanced skill and time, which can interrupt CPR if not performed efficiently.
Other routes, such as intracardiac or endotracheal drug delivery, are no longer recommended due to inconsistent absorption and increased complication risk.
Clinical Takeaway
- First-line: Attempt IV access promptly for drug administration.
- Alternative: Use IO access if IV is delayed or unsuccessful.
- Central access: Consider only in controlled, hospital environments by trained personnel.
- Avoid outdated routes like intracardiac or endotracheal drug delivery.
- Above all, ensure that vascular access efforts do not interrupt CPR or defibrillation.
Vasopressor Medications in Adult Cardiac Arrest
Vasopressors, particularly epinephrine, remain a cornerstone of pharmacologic management during cardiac arrest. Their primary role is to improve coronary and cerebral perfusion by increasing systemic vascular resistance, thereby enhancing the likelihood of return of spontaneous circulation (ROSC). However, while epinephrine increases ROSC and short-term survival, evidence for improved long-term neurological outcomes remains limited.
| # | Key Recommendation | Summary |
| 1 | Administer epinephrine for adult patients in cardiac arrest. | Epinephrine improves ROSC and short-term survival but not necessarily neurological outcomes. |
| 2 | Give 1 mg of epinephrine every 3–5 minutes during cardiac arrest. | Standard dosing remains best practice; high- or low-dose regimens offer no proven advantage. |
| 3 | In nonshockable rhythms (asystole or PEA), give epinephrine as soon as possible. | Early administration is associated with better chances of ROSC. |
| 4 | In shockable rhythms (VF/pVT), give epinephrine after initial defibrillation attempts have failed. | Focus on defibrillation first, then administer epinephrine if VF/pVT persists. |
| 5 | Do not substitute vasopressin for epinephrine, alone or in combination. | No clinical benefit shown compared with standard-dose epinephrine. |
| 6 | Avoid high-dose epinephrine during cardiac arrest. | High doses have not demonstrated improved outcomes and may increase post-arrest complications. |
Clinical Context
Epinephrine enhances coronary perfusion pressure, thereby improving the probability of ROSC, but studies show no significant improvement in survival to discharge or neurological recovery. Trials comparing different dosing strategies (high, low, or variable intervals) have not identified an optimal alternative to the standard 1 mg every 3–5 minutes. Vasopressin, although physiologically similar in its vasoconstrictive effect, has no added benefit when used instead of or alongside epinephrine.
Clinical Takeaway
- Use epinephrine 1 mg every 3–5 minutes as standard ACLS practice.
- Prioritize timing: give early in nonshockable rhythms; after defibrillation in shockable rhythms.
- Do not use high-dose epinephrine or vasopressin combinations—they provide no added survival benefit.
- Continue focusing on high-quality CPR, defibrillation, and addressing reversible causes, as these remain the strongest predictors of survival.
Non-vasopressor Medications During Cardiac Arrest
When high-quality CPR, defibrillation, and vasopressor therapy (e.g., epinephrine) fail to achieve ROSC, non-vasopressor medications may be considered. These include antiarrhythmics, metabolic agents, and steroids. However, evidence for improved survival or neurological recovery with most of these agents remains limited or absent.
| # | Key Recommendation | Summary |
| 1 | Amiodarone or lidocaine may be considered for VF/pVT unresponsive to defibrillation. | Both drugs can improve short-term outcomes such as ROSC, but neither improves survival to discharge. |
| 2 | The use of β-blockers, bretylium, procainamide, or sotalol for refractory VF/pVT is of uncertain benefit. | Limited evidence; not routinely recommended. |
| 3 | The use of corticosteroids during cardiac arrest is of uncertain benefit. | No conclusive evidence supporting improved outcomes. |
| 4 | Routine administration of calcium is not recommended. | Should only be given for specific indications such as hyperkalemia or calcium-channel blocker toxicity. |
| 5 | Routine administration of sodium bicarbonate is not recommended. | Reserved for conditions like tricyclic antidepressant overdose or severe hyperkalemia. |
| 6 | Routine administration of magnesium is not recommended. | Only indicated for torsades de pointes or known hypomagnesemia. |
Clinical Context
Nonvasopressor drugs have long been used to supplement resuscitation efforts, particularly in refractory cardiac arrest.
- Amiodarone and lidocaine remain acceptable for VF/pVT when shocks and epinephrine fail, with modest benefit in achieving ROSC.
- Other agents such as β-blockers, steroids, magnesium, and sodium bicarbonate lack sufficient evidence for routine use and may cause harm if administered indiscriminately.
- These medications retain value only in specific reversible causes, such as electrolyte disturbances, drug toxicity, or torsades de pointes.
Clinical Takeaway
- Use amiodarone or lidocaine only for shock-refractory VF/pVT.
- Avoid routine use of calcium, sodium bicarbonate, magnesium, or steroids unless a clear indication exists.
- Focus on definitive interventions — high-quality CPR, timely defibrillation, and addressing reversible causes (the “Hs and Ts”).
- Remember: drug therapy is supportive, not definitive, in cardiac arrest management.
Adjuncts for Performance of CPR
Modern resuscitation emphasizes high-quality CPR as the foundation of survival. Adjuncts such as ETCO₂ monitoring, point-of-care ultrasound (POCUS), arterial lines, and blood gas analysis can provide additional physiologic data to optimize resuscitation efforts. However, the 2025 AHA Guidelines note that while some adjuncts may aid in ROSC detection and reversible cause identification, most require further evidence before routine implementation.
| # | Key Recommendation | Summary |
| 1 | A sudden rise in end-tidal CO₂ (ETCO₂) may be used to detect ROSC during CPR or when an organized rhythm appears. | ETCO₂ monitoring is a valuable real-time indicator of perfusion and ROSC. |
| 2 | Use of point-of-care ultrasound (POCUS) to identify reversible causes during cardiac arrest is not well established. | Can assist with diagnosis (e.g., tamponade, pneumothorax) but may interrupt CPR if misused. |
| 3 | Use of echocardiography to assess cardiac function during resuscitation is not well established. | May assist in prognostication but should not delay chest compressions. |
| 4 | When available, use the highest feasible inspired oxygen concentration during CPR. | Maximizes arterial oxygen content while minimizing hypoxia risk. |
| 5 | Measuring arterial blood gases during CPR has uncertain benefit. | Limited value in guiding immediate management. |
| 6 | Using physiologic parameters (e.g., arterial pressure or ETCO₂) to monitor and optimize CPR quality may be reasonable when feasible. | Helps tailor compression depth and rate to improve perfusion. |
| 7 | Arterial pressure monitoring may be used to detect ROSC during compressions or organized rhythm. | Continuous arterial line monitoring can aid ROSC recognition. |
| 8 | Head-up CPR is not recommended except in clinical trials. | Insufficient evidence for benefit; should not be used in routine care. |
Clinical Context
Adjunctive tools can enhance resuscitation precision by providing real-time physiological feedback.
- ETCO₂ monitoring remains the most reliable non-invasive tool for assessing perfusion and early ROSC.
- POCUS and echocardiography can help identify reversible causes (e.g., tamponade, PE, hypovolemia), but must be used by trained personnel without interrupting compressions.
- Head-up CPR, though designed to improve cerebral perfusion, has not shown consistent benefit outside of research settings.
Clinical Takeaway
- Use ETCO₂ monitoring routinely when available to assess CPR quality and detect ROSC.
- Apply POCUS selectively and only by trained clinicians to avoid interruptions.
- Maintain 100% oxygen delivery during resuscitation.
- Monitor arterial pressure or ETCO₂ trends to optimize CPR performance.
- Avoid head-up CPR outside of controlled trials — it remains experimental.
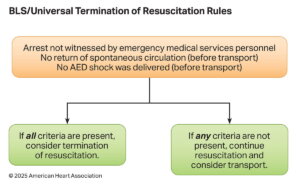
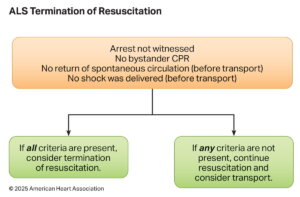
Termination of Resuscitative Measures
Termination of Resuscitation (TOR) rules guide when to stop resuscitation efforts in out-of-hospital cardiac arrest (OHCA) to balance patient survival potential with resource stewardship. The 2025 AHA Guidelines refine TOR criteria based on provider level (BLS, ALS, or Universal TOR) and emphasize a multimodal approach rather than reliance on a single factor
| # | Key Recommendation | Summary |
| 1 | BLS providers should apply the BLS TOR rule when ALS is unavailable or significantly delayed. | Guides BLS-only teams of determining when further resuscitation is futile. |
| 2 | ALS providers may use the ALS TOR rule to determine field termination for OHCA. | Allows ALS-trained personnel to end efforts when survival is highly unlikely. |
| 3 | In combined BLS/ALS systems, apply the Universal TOR (UTOR) rule. | Ensures consistency in tiered-response EMS systems. |
| 4 | For intubated patients, failure to achieve ETCO₂ >10 mmHg after 20 minutes of ALS resuscitation may support the decision to terminate. | ETCO₂ is a helpful, but not standalone, prognostic tool in a multimodal assessment. |
| 5 | In non-intubated patients, ETCO₂ values should not be used alone to decide termination. | Capnography is less reliable with bag-mask ventilation. |
Clinical Context
Out-of-hospital cardiac arrest has low survival rates, making it essential for EMS systems to distinguish futile efforts from potentially survivable cases.
The BLS, ALS, and Universal TOR rules provide structured, evidence-based guidance validated for specific levels of care. Key TOR criteria generally include unwitnessed arrest, no shocks delivered, and no ROSC achieved after sustained resuscitation efforts. ETCO₂ monitoring can supplement TOR decisions, but should never be the sole determinant — especially in non-intubated patients.
Clinical Takeaway
- Use validated TOR rules based on provider level (BLS, ALS, or Universal).
- ETCO₂ <10 mmHg after 20 minutes may support termination in intubated patients, but only within a broader clinical context.
- Avoid using ETCO₂ alone in non-intubated patients for TOR decisions.
- TOR rules should not be applied to special populations such as trauma, overdose, or in-hospital arrests.
- Applying TOR appropriately improves system efficiency while respecting patient dignity and clinical evidence.
Advanced Airway Placement During Resuscitation
Effective airway management is a critical component of cardiac arrest care. The 2025 AHA Guidelines emphasize that the choice, timing, and type of airway should be based on provider skill, system capability, and the patient’s condition. Both supraglottic airways (SGAs) and endotracheal intubation (ETI) are acceptable, provided they are performed safely and without interrupting CPR.
| # | Key Recommendation | Summary |
| 1 | Health care professionals performing intubation should undergo frequent practice or retraining. | Competency in ETI is essential to minimize complications and maximize success. |
| 2 | If airway placement will interrupt CPR, defer it until after initial CPR and defibrillation attempts. | Continuous compressions take priority over early airway insertion. |
| 3 | Use continuous waveform capnography to confirm and monitor ET tube placement. | Capnography is the gold standard for verifying correct airway placement. |
| 4 | EMS systems that perform intubation should have an ongoing quality improvement (QI) program to track success and complications. | Ensures accountability and continuous skill refinement. |
| 5 | In settings with low ETI success or minimal training, use a supraglottic airway (SGA). | SGAs provide a rapid, reliable alternative to intubation. |
| 6 | In settings with high ETI success and adequate training, either SGA or ETI is acceptable. | Choice depends on system proficiency and provider skill. |
| 7 | In-hospital teams trained in airway management may use either SGA or ETI. | Decision guided by expertise and patient context. |
| 8 | Either bag-mask ventilation (BMV) or an advanced airway strategy may be used during CPR. | Airway approach should match clinician skill and situation. |
| 9 | Once an advanced airway is in place, deliver 1 breath every 6 seconds (10 breaths/min) with continuous chest compressions. | Prevents hyperventilation and maintains oxygenation. |
Clinical Context
Airway management typically begins with basic manoeuvres such as bag-mask ventilation (BMV). Advanced airway placement—ETI or SGA—is then considered based on provider competence and patient response.
- Interruptions in chest compressions for airway insertion reduce perfusion and should be minimized.
- Waveform capnography remains the most reliable tool for confirming ET tube position and monitoring CPR effectiveness.
- Systems performing advanced airway interventions must implement ongoing QI programs to track placement success and complications.
- Airway selection should be context-driven, balancing benefits of definitive control with risks of delay or misplacement.
Clinical Takeaway
- Prioritize continuous, high-quality CPR over early intubation.
- Choose the airway (BMV, SGA, or ETI) based on provider skill and environment.
- Use capnography to confirm placement and monitor performance.
- Maintain 1 breath every 6 seconds with an advanced airway in place.
- Ensure frequent retraining and system-level quality monitoring to sustain safe, effective airway management practices.
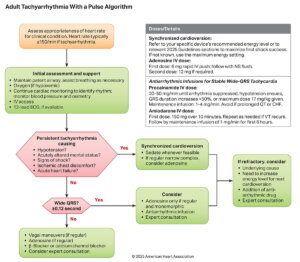
Wide-Complex Tachycardia (WCT)
A wide-complex tachycardia (WCT) is defined as a rhythm >150 bpm with a QRS duration ≥0.12 seconds. It can arise from ventricular origin (VT/VF) or supraventricular rhythms with aberrant conduction (e.g., bundle branch block or accessory pathway).
Because misdiagnosis can be life-threatening, WCT should be treated as ventricular tachycardia (VT) until proven otherwise.
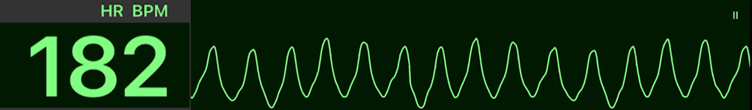
| # | Key Recommendation | Summary |
| 1 | Perform synchronized cardioversion for unstable WCT. | Immediate cardioversion is the treatment of choice for hemodynamic instability. |
| 2 | Perform synchronized cardioversion for stable WCT if vagal manoeuvres or medication are ineffective or contraindicated. | Use when pharmacologic or vagal interventions fail. |
| 3 | IV amiodarone, procainamide, or sotalol may be considered for WCT. | Appropriate for stable VT when cardioversion is not immediately needed. |
| 4 | IV adenosine may be considered in regular monomorphic WCT when rhythm diagnosis is uncertain. | Can aid in differentiating supraventricular from ventricular tachycardia. |
| 5 | Do not administer verapamil or diltiazem for WCT. | May worsen hypotension or precipitate cardiac arrest, especially in VT. |
| 6 | Do not give adenosine for unstable, irregular, or polymorphic WCT. | Contraindicated in torsades de pointes or AF with a bypass tract. |
Clinical Context
Wide-complex tachycardias can result from:
- Ventricular origin (VT/VF)
- Bundle branch block
- Accessory pathway conduction (e.g., WPW)
- Ventricular pacing
- Severe metabolic disturbances such as hyperkalemia or sodium channel blockade
Management depends on hemodynamic stability:
- Unstable patients: require immediate synchronized cardioversion.
- Stable patients: allow time for 12-lead ECG, IV access, and antiarrhythmic therapy.
- Pharmacologic choices should avoid calcium channel blockers (verapamil, diltiazem), which are contraindicated in WCT due to risk of cardiovascular collapse.
Clinical Takeaway
- Always assume WCT is ventricular until proven otherwise.
- Cardioversion is first-line for instability.
- Amiodarone, procainamide, or sotalol are preferred drugs for stable monomorphic VT.
- Adenosine can be used only in regular, monomorphic WCT of uncertain origin—not in polymorphic or irregular rhythms.
- Avoid verapamil, diltiazem, and adenosine in irregular or unstable WCT presentations.
Polymorphic Ventricular Tachycardia
Polymorphic ventricular tachycardia (pVT) is a wide-complex tachycardia of ventricular origin characterized by beat-to-beat variation in QRS morphology. The clinical significance lies not in its appearance but in the underlying QT interval — which determines whether the rhythm is torsades de pointes (QT prolonged) or ischemic polymorphic VT (normal QT). Because pVT often degenerates into ventricular fibrillation (VF), prompt unsynchronized defibrillation is critical for survival.
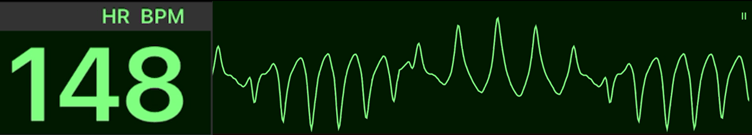
| # | Key Recommendation | Summary |
| 1 | Deliver immediate unsynchronized shock for sustained polymorphic VT. | Treat as VF; early defibrillation is lifesaving. |
| 2 | Administer magnesium for recurrent polymorphic VT associated with prolonged QT (torsades de pointes). | Stabilizes cardiac repolarization and suppresses early after-depolarizations. |
| 3 | IV lidocaine, amiodarone, and treatment of underlying ischemia may be considered for polymorphic VT with normal QT. | Focus on correcting myocardial ischemia and reducing irritability. |
| 4 | Do not use magnesium routinely for polymorphic VT with normal QT. | Provides no benefit in non-torsades polymorphic VT. |
Clinical Context
Polymorphic VT may present in several forms, but the QT interval determines the underlying mechanism and treatment approach:
- Prolonged QT (Torsades de Pointes): Often due to drugs, electrolyte imbalance, or genetic syndromes. Risk increases when QTc > 500 ms, especially with bradycardia.
- Normal QT: Commonly due to acute ischemia, catecholaminergic pVT, or toxic/metabolic causes such as digitalis toxicity or short QT syndrome.
Because evidence is largely based on case studies, therapy focuses on immediate shock delivery and addressing reversible causes (e.g., correcting electrolytes, stopping offending drugs, managing ischemia).
Clinical Takeaway
- Treat all sustained polymorphic VT as VF — deliver immediate defibrillation.
- For torsades de pointes (prolonged QT): give IV magnesium and correct reversible causes (hypokalemia, drug toxicity).
- For normal QT polymorphic VT: manage with antiarrhythmics (lidocaine, amiodarone) and treat ischemia.
- Do not give magnesium unless the QT interval is prolonged.
- Always address precipitating factors — ischemia, electrolyte abnormalities, or drug effects — to prevent recurrence.
Regular Narrow-Complex Tachycardia
Regular narrow-complex tachycardia (NCT) refers to supraventricular rhythms with a QRS duration <0.12 seconds and a regular rate, typically caused by AV nodal re-entry (AVNRT), AV re-entrant tachycardia (AVRT), atrial flutter, or focal atrial tachycardia.
The 2025 AHA Guidelines reaffirm a stepwise management approach, prioritizing non-pharmacologic measures first and escalating to medications or cardioversion as needed.

| # | Key Recommendation | Summary |
| 1 | Perform vagal manoeuvres for acute treatment of regular NCT. | First-line intervention; effective in terminating up to 25% of SVTs. |
| 2 | Administer adenosine for acute management of regular NCT. | Rapidly terminates reentrant tachycardias involving the AV node. |
| 3 | Perform synchronized cardioversion for unstable NCT. | Immediate therapy for patients with hypotension, shock, chest pain, or altered mentation. |
| 4 | Perform synchronized cardioversion for stable NCT if vagal manoeuvres or medications fail or are contraindicated. | Used when pharmacologic therapy is ineffective or inappropriate. |
| 5 | IV diltiazem or verapamil can be effective for stable regular NCT. | Calcium channel blockers slow AV nodal conduction and terminate re-entrant rhythms. |
| 6 | IV beta-blockers (e.g., metoprolol, esmolol) are reasonable for stable NCT. | Useful when calcium channel blockers are contraindicated or ineffective. |
Clinical Context
Most narrow-complex tachycardias are not immediately life-threatening, allowing time for a structured diagnostic and therapeutic approach:
- Vagal manoeuvres stimulate the vagus nerve, transiently slowing AV conduction and potentially terminating AVNRT or AVRT.
- Adenosine remains the drug of choice for rapid termination of regular NCTs and can also help diagnose the underlying rhythm.
- In hemodynamically unstable cases, immediate synchronized cardioversion is indicated.
- For stable but persistent NCT, calcium channel blockers or beta-blockers may be used to control rate or restore sinus rhythm.
- Always rule out secondary causes of tachycardia (e.g., fever, sepsis, hypovolemia) before initiating electrical or pharmacologic intervention.
Clinical Takeaway
- Begin with vagal manoeuvres, progressing to adenosine if ineffective.
- Cardioversion is required for unstable patients or refractory tachycardia.
- Verapamil, diltiazem, or beta-blockers are appropriate alternatives for stable cases unresponsive to adenosine.
- Avoid unnecessary cardioversion if the tachycardia is compensatory (secondary to another condition).
- With appropriate interventions, conversion success rates exceed 95%, making regular NCT one of the most treatable cardiac arrhythmias.
Atrial Fibrillation or Flutter With Rapid Ventricular Response (RVR) – Part 1
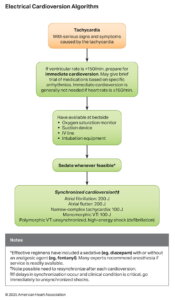
Atrial fibrillation (AF) and atrial flutter are among the most common tachyarrhythmias encountered in adults. AF is a disorganized atrial rhythm caused by multiple re-entrant circuits, while atrial flutter is a more organized macro-re-entrant rhythm. Both may lead to rapid ventricular response (RVR), resulting in decreased cardiac output, hypotension, or myocardial ischemia. The 2025 AHA Guidelines reaffirm that hemodynamic instability caused by AF/flutter with RVR requires immediate electrical cardioversion.
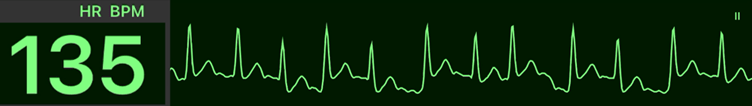
| # | Key Recommendation | Summary |
| 1 | Perform immediate synchronized cardioversion for hemodynamically unstable AF/flutter with RVR. | Indicated for hypotension, shock, chest pain, or heart failure caused by rapid rates. |
| 2 | Perform urgent direct-current cardioversion in new-onset AF/flutter with RVR and acute coronary syndrome (ACS) causing ischemia or poor rate control. | Prompt rhythm restoration prevents further ischemic injury. |
| 3 | For AF, use biphasic cardioversion starting at ≥200 J, increasing energy if needed. | Optimal energy setting depends on the device and patient factors. |
| 4 | The benefit of double synchronized cardioversion as an initial strategy for AF is uncertain. | Not routinely recommended; evidence limited. |
| 5 | For atrial flutter, start synchronized cardioversion at 200 J, increasing if initial shock fails. | Flutter typically requires lower energy than AF but responds well to 200 J with biphasic waveforms. |
| 6 | The usefulness of double synchronized cardioversion as a rescue treatment for shock-refractory AF is uncertain. | Reserved for research or last-resort clinical judgment. |
Clinical Context
Electrical cardioversion is the treatment of choice for unstable AF/flutter with RVR.
Success depends not only on energy level but also on technical and patient-specific factors, including:
- Pad size and placement (anterolateral or anteroposterior configuration)
- Minimizing impedance through good skin contact and proper gel use
- Shock waveform (biphasic preferred)
- Timing with the R-wave (synchronized shock)
Before cardioversion, clinicians must assess rhythm duration and anticoagulation status:
- For AF/flutter >48 hours or unknown duration, cardioversion without anticoagulation increases thromboembolic risk.
- For <48 hours or when hemodynamic instability is present, urgent cardioversion is appropriate with subsequent anticoagulation planning.
Clinical Takeaway
- Unstable AF/flutter with RVR: Perform immediate synchronized cardioversion.
- Stable AF/flutter with ACS and ischemia: Urgent cardioversion recommended.
- Energy selection: Start with ≥200 J biphasic, escalate if unsuccessful.
- Double cardioversion: Currently unsupported by evidence; not routine.
- Always consider anticoagulation status, shock technique, and underlying causes (ischemia, structural heart disease, electrolyte imbalance).
Atrial Fibrillation and Flutter With Rapid Ventricular Response (RVR): Part 2 — Medical Therapies
The 2025 AHA Guidelines outline pharmacologic options for rate control and rhythm management in AF/flutter with RVR.The choice of therapy depends on hemodynamic stability, presence of preexcitation, and ventricular function.
The primary goal in the acute setting is to slow the ventricular rate, restore hemodynamic stability, and prevent deterioration into more unstable rhythms such as ventricular fibrillation (VF).
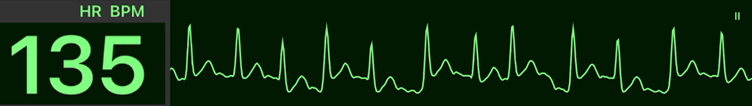
| # | Key Recommendation | Summary |
| 1 | Administer IV beta-blockers or nondihydropyridine calcium channel blockers (diltiazem, verapamil) to slow the ventricular rate in AF/flutter without preexcitation. | First-line for acute rate control in hemodynamically stable patients. |
| 2 | IV amiodarone can be useful for rate control in critically ill patients with AF/flutter without preexcitation. | Preferred when hypotension, shock, or heart failure precludes beta-blocker or calcium channel blocker use. |
| 3 | Avoid digoxin, beta-blockers, calcium channel blockers, and IV amiodarone in preexcited AF/flutter (WPW). | These agents can accelerate conduction through the accessory pathway, precipitating VF. |
| 4 | Avoid calcium channel blockers and IV beta-blockers in systolic heart failure or decompensated LV dysfunction. | May worsen cardiac output and cause hemodynamic collapse. |
Clinical Context
- Rate control is the initial focus in most patients with AF/flutter and RVR.
- Beta-blockers (metoprolol, esmolol) and calcium channel blockers (verapamil, diltiazem) effectively slow AV nodal conduction.
- Amiodarone serves a dual purpose — it can assist with both rate and rhythm control, especially in critically ill or heart failure patients where other agents are contraindicated.
- Preexcited AF/flutter (e.g., Wolff–Parkinson–White syndrome) is exceptional because AV nodal blocking agents can paradoxically increase ventricular rate by facilitating accessory pathway conduction, potentially leading to VF.
- In patients with decompensated heart failure, nondihydropyridine calcium channel blockers (verapamil, diltiazem) and IV beta-blockers should be avoided, as they can worsen cardiac output and hypotension.
Clinical Takeaway
- Stable AF/flutter with RVR: Start with IV beta-blocker or calcium channel blocker for rate control.
- Critically ill or heart failure patients: Use IV amiodarone if other agents are contraindicated.
- Preexcited AF/flutter (WPW): Avoid AV nodal blockers — these can cause ventricular fibrillation.
- Decompensated heart failure: Avoid verapamil, diltiazem, and IV beta-blockers.
- Always tailor drug selection to hemodynamic status, underlying pathology, and rhythm mechanism.
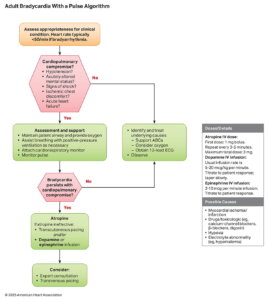
Initial Management of Bradycardia
Bradycardia is defined as a ventricular rate below 50 beats per minute, though it may be normal in athletes or during rest and sleep. In the emergency setting, the focus is on identifying symptomatic bradycardia that causes hemodynamic compromise, such as hypotension, altered mental status, chest pain, or signs of shock. The 2025 AHA Guidelines emphasize rapid recognition, treatment of reversible causes, and prompt intervention to restore perfusion.
| # | Key Recommendation | Summary |
| 1 | Evaluate and treat reversible causes in all patients with symptomatic bradycardia. | Address underlying factors such as hypoxia, ischemia, hypothermia, or drug toxicity. |
| 2 | Administer atropine for bradycardia causing hemodynamic compromise. | First-line medication to increase heart rate and improve perfusion. |
| 3 | Use temporary transvenous pacing for persistent unstable bradycardia refractory to medical therapy. | Provides reliable rate control when drugs fail. |
| 4 | If unresponsive to atropine, use IV adrenergic agonists (e.g., epinephrine, dopamine) or transcutaneous pacing while preparing for transvenous pacing. | Serves as a bridge to definitive pacing. |
| 5 | Consider immediate pacing if the patient is unstable with high-degree AV block and IV/IO access is unavailable. | Life-saving measure when drug delivery is delayed or impossible. |
Clinical Context
Bradycardia can range from benign (e.g., athlete’s heart) to life-threatening, depending on its cause and impact on perfusion.
- Reversible causes include myocardial ischemia, hypoxia, electrolyte imbalances, acidosis, hypothermia, or medication toxicity (e.g., beta-blockers, calcium channel blockers).
- Symptomatic bradycardia presents with dizziness, hypotension, chest discomfort, heart failure, or syncope due to reduced cardiac output.
- The management algorithm prioritizes maintaining perfusion while addressing underlying causes, beginning with atropine, progressing to catecholamines or pacing if needed.
Clinical Takeaway
- Step 1: Identify and treat reversible causes (e.g., hypoxia, ischemia, electrolyte imbalance).
- Step 2: If unstable, give atropine 1 mg IV every 3–5 min (max 3 mg).
- Step 3: If atropine ineffective, use epinephrine (2–10 µg/min) or dopamine (5–20 µg/kg/min) infusion, or initiate transcutaneous pacing.
- Step 4: Prepare for transvenous pacing if bradycardia persists.
- Step 5: For high-degree AV block, consider immediate pacing if IV/IO access is delayed.
- Always assess for hemodynamic compromise, support oxygenation and perfusion, and correct underlying causes.
Advance Your ACLS Skills With HSTCSA
Understanding the 2025 AHA Guidelines is an essential component of Advanced Cardiovascular Life Support (ACLS) training. At the Health & Safety Training Centre of South Africa (HSTCSA), we deliver internationally aligned AHA ACLS Courses for doctors, nurses, and paramedics — combining evidence-based education, hands-on simulation, and South African clinical context.
Book your ACLS course today to gain confidence in managing bradycardia, tachyarrhythmias, and cardiac arrest emergencies with the latest evidence-based guidance.
Book Your ACLS Course Here →